Subtotal: $337.80
Opana 40mg
$70.00
The Opana 40mg tablet was an extended-release (ER) version of the opioid painkiller oxymorphone, which is no longer on the market in the U.S. at the request of the Food and Drug Administration (FDA). The description below applies to the formulation as it existed before its voluntary market removal by manufacturer Endo Pharmaceuticals in 2017 due to risks of misuse and abuse.
Key characteristics of Opana ER 40mg
- Active ingredient: Oxymorphone hydrochloride, a Schedule II controlled substance with a high potential for abuse.
- Purpose: To provide continuous, long-lasting pain relief, with effects lasting up to 12 hours. It was not intended for as-needed (PRN) pain relief.
- Formulation: The original formula was easily crushed, making it susceptible to abuse via snorting or injection, which could lead to a rapid, fatal overdose. A reformulated version was later introduced to be more difficult to crush.
- Physical description (Reformulated ER tablet): It was an orange, oval tablet. The 40mg strength was one of several available dosages, including 5mg, 10mg, and 20mg.
- Special instructions: Tablets had to be swallowed whole. Chewing, crushing, or dissolving them would destroy the extended-release mechanism, causing a rapid release of the opioid and a risk of overdose. It was also not to be taken with alcohol, which could increase plasma levels of the drug and cause an overdose.
In 2017, the U.S. Food and Drug Administration (FDA) requested that Endo Pharmaceuticals remove Opana ER from the market, citing the high potential for abuse. The FDA’s decision was based on evidence that despite the tamper-resistant reformulation, the drug continued to be significantly abused, with public health consequences. Following the request, Endo voluntarily removed the product.
Be the first to review “Opana 40mg” Cancel reply
Related products
Painkillers
Painkillers
Painkillers
Painkillers
Painkillers
Painkillers
Painkillers

 Modafinil Tablets
Modafinil Tablets  Oxycontin
Oxycontin 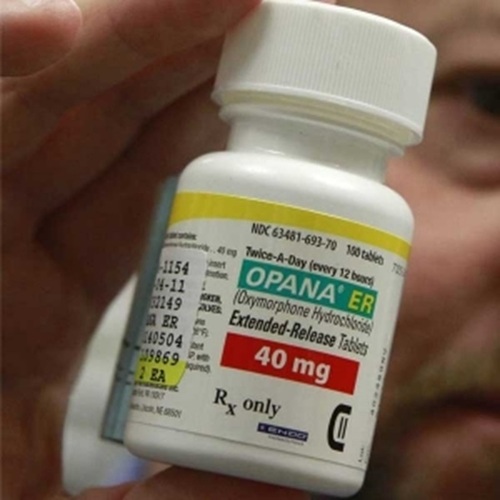


Reviews
There are no reviews yet.